Introduction
Follicular lymphoma (FL) is the most common subtype of indolent non-Hodgkin lymphoma (NHL), originating in follicular central B cells, and accounts for approximately 22% of all newly diagnosed cases of NHL. Chemo-immunotherapy is the standard treatment option for FL. Unfortunately, the progression of the disease to relapsed or refractory FL (r/r FL) is still inevitable, which raised concern about subsequent effective treatments. Several δ isoform of phosphatidylinositol 3-kinase (PI3K-δ) inhibitors have ever been approved for r/r FL after ≥2 lines of prior therapies. However, most of their indications for r/r FL were withdrawal for increased risk of death and serious side effects. The latest data from the Chinese phase II clinical trial of linperlisib (Trial registration ID: NCT04370405), a novel oral PI3Kδ-selective inhibitor, in patients with r/r FL who had received at least two systemic therapies were presented. In this trial, linperlisib demonstrated compelling efficacy and was generally well-tolerated in the treatment of r/r FL patients after ≥2 prior systemic therapies. Linperlisib was approved by China National Medical Products Administration (NMPA) for adult r/r FL previously treated with at least two system therapies in November 2022. The ≥3 lines of treatment options are still unmet for the r/r FL. In this setting, this subgroup analysis of the linperlisib phase II trial aimed to evaluate outcomes of linperlisib in later-line treatment of r/r FL.
Methods
This study included all the patients with r/r FL who had received at least two systemic therapies previously enrolled in the open-label, single-arm, phase II trial (linperlisib, 80mg, po, q.d., in a 28-day cycle), with overall response rate (ORR) as the primary endpoint. In this subgroup analysis, the patients were divided into two groups according to the treatment lines they received: >3 prior lines of treatment and ≤3 prior lines of treatment. All of the statistical analyses were descriptive.
Results
A total of 84 r/r FL patients from 25 sites in China from April 2019 to September 2020 were enrolled in this trial, with a cutoff date of September 30, 2021. The ORR was 79.8%, with statistical significance in a heavily pretreated FL patient population with a median of 4 prior therapies, with a well-tolerated safety profile. A total of 43 patients received >3 prior lines of treatment, while 41 patients received ≤3 prior lines of treatment.
The median age between the two groups was similar (range: 29.0 - 78.0, 52.0 years for >3 prior lines vs. 48.0 years for ≤3 prior lines). The majority of patients had Ann Arbor staging III-IV (40 for >3 prior lines vs. 34 for ≤3 prior lines). The median number of prior regimens was 4 for >3 prior lines and 3 for ≤3 prior lines.
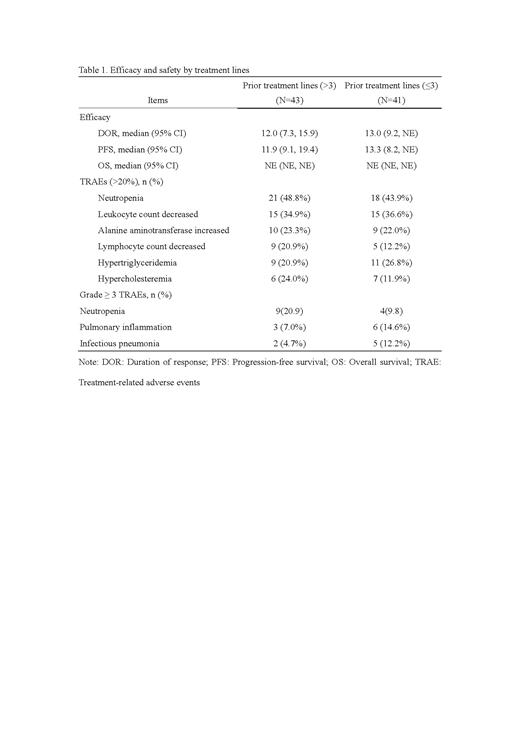
Overall, there were 67 patients achieved responses, with ORR of 83.7% (36, 95%CI:69.3, 93.2) for >3 prior lines vs. 75.6% (31, 95% CI: 59.7, 87.6) for ≤3 prior lines based on independent review committee assessment. Compared with patients who received ≤3 prior lines, patients received ≤3 prior lines achieved higher disease control rate (41[95.3] vs. 37 [90.2%]). The median DOR was 12 months (95% CI: 7.3, 15.9) for >3 prior lines vs. 13 months (95% CI: 9.2, NE). The median PFS was 11.9 months (95% CI: 9.1,19.4) for >3 prior lines vs. 13.3 months (95% CI: 8.2, NE) for ≤3 prior lines. The OS of both groups was not reached.
The most common treatment-related adverse events (≥20%, TRAEs) were neutropenia (21 [48.8%] for >3 prior lines vs. 18 [43.9%] for ≤3 prior lines), leukocyte count decreased (15 [34.9%] vs. 15 [36.6%]), alanine aminotransferase increased (10 [23.3%] vs. 9 [22.0%]), lymphocyte count decreased (9 [20.9%] vs. 5 [12.2%]), hypertriglyceridemia (9 [20.9%] vs. 11 [26.8%]), and hypercholesteremia (6 [24.0%] vs. 7 [11.9%]). The most common grade ≥3 TRAEs (≥10%) were neutropenia (9 [20.9%] vs. 4 [9.8%]), pulmonary inflammation (3 [7.0%] vs. 6 [14.6%]), infectious pneumonia (2 [4.7%] vs. 5 [12.2%]).
Conclusions
This subgroup analysis revealed that linperlisib could achieve benefit in r/r FL patients who received at least 2 prior system therapies regardless of treatment lines. The AEs were well-tolerated. Further trials with large-scale samples were warranted.
Disclosures
Fu:Shanghai Changzheng Hospital: Other: WJF is a former staff of Shanghai Changzheng Hospital and now is a staff of Shanghai Fourth People's Hospital affiliated to Tongji University. ; Takeda Pharmaceutical Company Limited.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal